Is Thaumatin Powder Safe to Consume?
Thaumatin powder has been gaining popularity as a natural sweetener with intense sweetening properties. Derived from the Thaumatococcus daniellii plant native to West Africa, this protein-based sweetener is approximately 2,000 times sweeter than sucrose while containing virtually no calories. As consumers increasingly seek alternatives to artificial sweeteners and sugar, questions about the safety and applications of thaumatin powder have become more prevalent. This article explores the safety profile, regulatory status, and potential benefits of thaumatin powder as a sweetening agent in food and beverages.
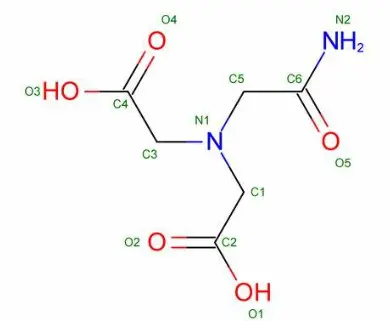
What Are the Health Benefits of Thaumatin Powder?
Calorie-Free Alternative to Sugar
Thaumatin powder offers a remarkable solution for individuals seeking to reduce their sugar intake without sacrificing sweetness in their diet. As a protein-based sweetener, thaumatin powder provides intense sweetness with virtually no caloric contribution. This makes it an excellent option for those managing diabetes, following weight management programs, or simply trying to reduce their overall sugar consumption. Unlike many artificial sweeteners that may leave an unpleasant aftertaste, thaumatin powder has a clean, sweet taste profile with a slight licorice-like quality that develops slowly and lingers longer than sugar. Research indicates that incorporating thaumatin powder into a balanced diet can help reduce total caloric intake while maintaining satisfaction with sweet foods, potentially contributing to better glycemic control and weight management outcomes over time.
Anti-inflammatory and Antioxidant Properties
Beyond its sweetening capabilities, thaumatin powder has demonstrated promising anti-inflammatory and antioxidant properties in preliminary studies. The protein structure of thaumatin contains compounds that may help neutralize harmful free radicals in the body and reduce oxidative stress. Some research suggests that thaumatin powder may help modulate inflammatory responses at the cellular level, potentially offering protective effects against chronic inflammation associated with various health conditions. While more clinical studies are needed to fully understand these potential benefits, the current evidence indicates that thaumatin powder may provide additional health advantages beyond its primary function as a sweetener. Regular consumption of thaumatin powder in appropriate amounts could potentially contribute to overall antioxidant intake, supporting the body's natural defense systems against oxidative damage and inflammation.
Dental Health Benefits
Unlike sugar and many other sweeteners, thaumatin powder appears to be non-cariogenic, meaning it doesn't contribute to tooth decay or cavity formation. The molecular structure of thaumatin powder prevents it from being metabolized by the oral bacteria that typically convert sugars into acids that erode tooth enamel. This property makes thaumatin powder a dentist-friendly sweetening option, particularly for products like chewing gum, mouthwash, toothpaste, and children's medications. Some dental professionals have even begun recommending thaumatin powder as a preferred sweetener for patients with high caries risk or those undergoing orthodontic treatment. The potential dental benefits of thaumatin powder represent a significant advantage over traditional sugars and even some alternative sweeteners that may still contribute to dental issues despite being lower in calories.
How Is Thaumatin Powder Regulated for Food Safety?
FDA Status and Global Regulatory Approvals
Thaumatin powder has undergone extensive regulatory review worldwide and has secured approval from numerous food safety authorities. In the United States, the FDA has granted thaumatin powder GRAS (Generally Recognized As Safe) status, allowing its use as a flavor enhancer and sweetener in various food categories. The European Food Safety Authority (EFSA) has also extensively evaluated thaumatin powder and approved it as food additive E957 for use in the European Union. Japan, Australia, New Zealand, and many other countries have similarly approved thaumatin powder for food applications. These regulatory approvals follow rigorous scientific assessment of toxicological data, manufacturing processes, and potential dietary exposure levels. The consistent safety determinations across different regulatory bodies worldwide provide strong evidence for the safety profile of thaumatin powder when used as directed in food products.
Safety Studies and Toxicological Assessment
Numerous safety studies have been conducted on thaumatin powder to assess its potential for toxicity or adverse effects. Acute and chronic toxicity studies, mutagenicity testing, and reproductive toxicity evaluations have consistently shown no significant safety concerns with thaumatin powder consumption. As a naturally occurring protein, thaumatin powder is metabolized through normal protein digestion pathways, breaking down into constituent amino acids that are utilized by the body. The ADI (Acceptable Daily Intake) for thaumatin powder has been established as "not specified" by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), indicating that consumption of thaumatin powder within normal use levels presents no appreciable health risk. This favorable safety profile has been reinforced by decades of safe use in various food products and the absence of significant adverse event reports despite widespread consumption of thaumatin powder in commercial products.
Allergenicity Considerations and Labeling Requirements
As with any protein-based food ingredient, potential allergenicity of thaumatin powder has been carefully evaluated by regulatory authorities. While severe allergic reactions to thaumatin powder are extremely rare, manufacturers are required to list it on product labels in accordance with food allergen labeling regulations in most countries. The protein structure of thaumatin powder is distinct from common allergens such as peanuts, tree nuts, milk, eggs, soy, wheat, fish, and shellfish, which may explain its low allergenic potential. Nevertheless, individuals with known protein sensitivities or multiple food allergies should exercise caution when trying products containing thaumatin powder for the first time. Food manufacturers using thaumatin powder must comply with specific labeling requirements that vary by country, typically listing it either by name or by its E-number (E957) in regions where this system is used.
What Are the Applications and Limitations of Thaumatin Powder?
Food and Beverage Industry Usage
Thaumatin powder has found widespread application across the food and beverage industry, where its intense sweetening power and flavor-modifying properties make it particularly valuable. In beverages, thaumatin powder is often used in combination with other sweeteners to create balanced sweetness profiles while reducing overall sugar content. The dairy industry utilizes thaumatin powder in yogurts, ice creams, and flavored milk products to enhance sweetness while improving mouthfeel and masking undesirable aftertastes from other ingredients. Confectionery manufacturers incorporate thaumatin powder into candies, chocolates, and chewing gums to achieve sweetness with minimal caloric impact. Perhaps most notably, thaumatin powder excels as a flavor enhancer that can boost perception of fruity, chocolate, and vanilla notes while simultaneously reducing bitter or astringent notes in various food formulations. This multifunctional profile makes thaumatin powder an exceptionally versatile ingredient for food developers seeking natural, clean-label solutions to sweetening challenges.
Pharmaceutical and Nutraceutical Applications
Beyond conventional food applications, thaumatin powder has gained significant traction in pharmaceutical and nutraceutical formulations. The pharmaceutical industry values thaumatin powder for its ability to mask the bitter taste of many medications, particularly important for pediatric formulations, elderly patients, or others who struggle with medication compliance due to unpleasant taste. Chewable tablets, syrups, and oral suspensions frequently contain thaumatin powder as a taste-improving agent. In the nutraceutical sector, thaumatin powder helps improve the palatability of protein supplements, herbal preparations, and vitamin formulations that may otherwise have challenging flavor profiles. Manufacturers of sports nutrition products increasingly turn to thaumatin powder as a natural sweetening option that aligns with clean-label preferences while avoiding the caloric load of traditional sweeteners. As interest in functional foods and personalized nutrition continues to grow, thaumatin powder's role in these specialized health products appears poised for further expansion.
Taste Profile and Technical Limitations
While thaumatin powder offers many advantages, food formulators must consider its unique taste characteristics and certain technical limitations. The sweetness of thaumatin powder develops more slowly than sucrose and lingers longer, which can be either advantageous or challenging depending on the application. Some consumers detect subtle licorice-like or menthol notes in thaumatin powder at higher concentrations, which may not be desirable in all product types. From a technical perspective, thaumatin powder's stability can be affected by prolonged exposure to high temperatures (above 100°C), making it less suitable for certain high-heat cooking applications. Additionally, thaumatin powder works synergistically with some sweeteners but may create unexpected taste interactions with others, requiring careful formulation work. Cost considerations also come into play, as thaumatin powder typically commands a premium price compared to conventional sweeteners, though this is often offset by the extremely small quantities required due to its intense sweetness. Despite these limitations, skilled food technologists have successfully incorporated thaumatin powder into an increasingly diverse range of products by understanding and working with its unique properties.

Conclusion
Thaumatin powder emerges as a safe, natural sweetener with regulatory approval worldwide. Its intense sweetness, zero-calorie profile, and additional benefits for dental health and potential anti-inflammatory properties make it an attractive alternative to artificial sweeteners. While some taste and technical limitations exist, thaumatin powder continues to gain popularity across food, beverage, and pharmaceutical applications. For consumers seeking natural sweetening options, thaumatin powder represents a well-studied, safe choice.
Shaanxi Yuantai Biological Technology Co., Ltd. (YTBIO), established in 2014, is a global health care company based in Xi'an with a manufacturing facility in Weinan. We specialize in health food ingredients (such as Herbal Extracts, Magnesium Threonate, and Creatine Monhydrate) and cosmetic ingredients (including Sponge Spicule, Retinol, Glutathione, and Arbutin). We work with partners in Europe, America, Southeast Asia, and Korea. With a warehouse in Rotterdam for EU distribution and plans for U.S. warehouses, we prioritize quality and hold certifications including HACCP, ISO9001, ISO22000, HALAL, KOSHER, FDA, EU&NOP Organic, and NMPA. We also assist Korean clients with KFDA registration. Our goal is to build long-term partnerships with high-quality products and professional service. For inquiries, contact us at sales@sxytorganic.com or +86-029-86478251 / +86-029-86119593.
References
1. Faus, I. (2000). Recent developments in the characterization and biotechnological production of sweet-tasting proteins. Applied Microbiology and Biotechnology, 53(2), 145-151.
2. Kant, R. (2005). Sweet proteins—Potential replacement for artificial low calorie sweeteners. Nutrition Journal, 4(1), 5.
3. Nabors, L. O. B. (Ed.). (2016). Alternative Sweeteners (4th ed.). CRC Press, Taylor & Francis Group.
4. Joint FAO/WHO Expert Committee on Food Additives. (2006). Evaluation of certain food additives: sixty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, 934.
5. McClements, D. J., & Decker, E. A. (2021). Designing functional foods: Measuring and controlling food structure breakdown and nutrient absorption. Woodhead Publishing.
6. Kinghorn, A. D., Compadre, C. M., & Pezzuto, J. M. (2002). Sweetening agents of plant origin. In B. Seidman & P. Shaw (Eds.), Functional Foods: Biochemical and Processing Aspects (Vol. 2, pp. 203-230). CRC Press.
_1737093401309.png)
