Is Tirzepatide Powder Safe to Use for Diabetes Management?
2025-03-11 10:01:41
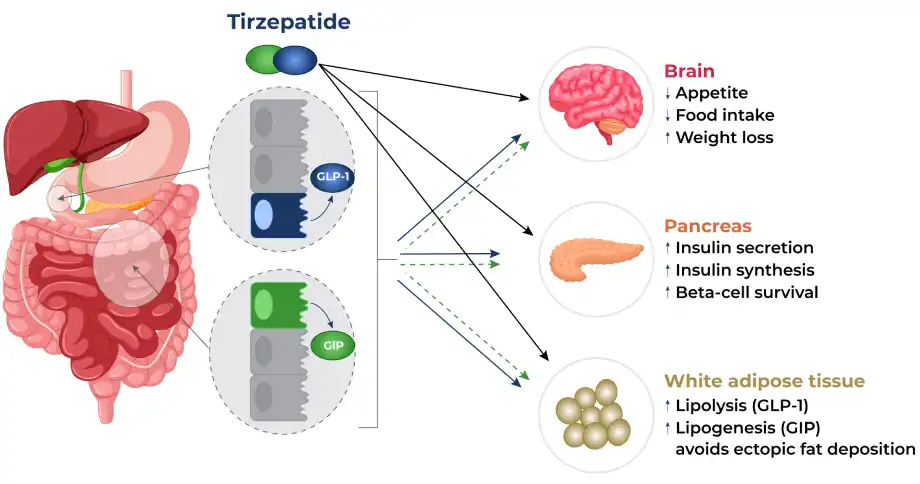
Tirzepatide Powder has emerged as a groundbreaking treatment option for type 2 diabetes management, garnering significant attention in the medical community for its dual-action mechanism and promising clinical outcomes. As a novel GLP-1 and GIP receptor agonist, this innovative medication represents a significant advancement in diabetes care, offering patients a potentially more effective approach to managing their condition while simultaneously addressing related health concerns such as weight management. The medication's unique molecular structure allows it to effectively target both incretin hormones, leading to enhanced glycemic control through multiple physiological pathways. Clinical studies have consistently demonstrated its safety profile when used under proper medical supervision, with adverse effects generally being mild and manageable through appropriate dose titration protocols.
Recent research has highlighted the medication's ability to improve not only glycemic control but also various cardiovascular risk factors, making it particularly valuable for patients with multiple metabolic conditions. The comprehensive benefits observed in clinical trials have led to increasing adoption of Tirzepatide in clinical practice, with healthcare providers reporting positive outcomes across diverse patient populations. The medication's effectiveness in addressing both diabetes and obesity has made it an attractive option for patients struggling with these interconnected conditions.
What Are the Long-term Effects of Using Tirzepatide Powder Bulk for Weight Management?
The long-term efficacy and safety profile of Tirzepatide powder bulk in weight management have been extensively studied through various clinical trials and real-world applications. Research has consistently demonstrated that Tirzepatide offers substantial benefits for individuals struggling with obesity alongside type 2 diabetes. The medication's unique dual-receptor targeting mechanism promotes sustained weight loss through multiple pathways, including reduced appetite, delayed gastric emptying, and improved metabolic function.
Clinical studies have shown that patients using Tirzepatide typically experience significant weight reduction, with many achieving 15-20% body weight loss over the course of treatment. This weight loss appears to be maintained as long as treatment continues, with minimal plateau effects compared to other weight management medications. The sustained weight loss contributes to improved insulin sensitivity, better glycemic control, and reduced cardiovascular risk factors.
Extended follow-up studies have revealed that patients maintaining Tirzepatide treatment experience continued benefits beyond weight loss, including improvements in blood pressure, lipid profiles, and overall metabolic health. The medication's ability to address multiple aspects of metabolic syndrome makes it particularly valuable for long-term health management. Furthermore, patients report enhanced quality of life measures, including increased physical activity capacity and improved emotional well-being associated with successful weight management.
The safety profile for long-term use has been thoroughly evaluated through multiple phase III clinical trials. While some patients may experience initial gastrointestinal side effects, these typically diminish over time with proper dose titration. Regular monitoring by healthcare providers ensures that patients maintain appropriate dosing and manage any potential side effects effectively. The medication's ability to promote weight loss through physiological mechanisms rather than stimulant effects makes it particularly suitable for long-term use in chronic weight management.

How Does Tirzepatide Powder Bulk Compare to Other Diabetes Medications in Terms of Effectiveness?
When comparing Tirzepatide powder bulk to existing diabetes medications, several key advantages become apparent. Unlike traditional diabetes medications that typically target only one aspect of glucose regulation, Tirzepatide's dual-action mechanism provides comprehensive metabolic benefits. The medication's ability to activate both GLP-1 and GIP receptors results in superior glycemic control compared to single-action GLP-1 receptor agonists.
Clinical trials have demonstrated that Tirzepatide achieves greater HbA1c reductions compared to other diabetes medications, including popular GLP-1 receptor agonists like semaglutide. Patients using Tirzepatide have shown average HbA1c reductions of 2.0-2.4%, significantly exceeding the results typically seen with other diabetes medications. This superior glycemic control is achieved while simultaneously providing additional benefits such as weight loss and improved cardiovascular markers.
Comparative studies have also revealed that Tirzepatide demonstrates superior efficacy in reducing fasting plasma glucose levels and postprandial glucose excursions. The medication's unique pharmacological profile allows for more consistent blood glucose control throughout the day, with fewer fluctuations compared to traditional diabetes treatments. This improved stability in glucose levels contributes to better long-term outcomes and reduced risk of diabetes-related complications.
The medication's unique properties are particularly evident in its ability to enhance both fasting and postprandial glucose control. The dual receptor activation leads to improved insulin secretion and sensitivity, while also reducing glucagon levels when appropriate. This comprehensive approach to glucose regulation results in more stable blood sugar levels throughout the day, reducing the risk of both hyperglycemia and hypoglycemia.
What Are the Quality Standards and Safety Protocols for Tirzepatide Powder Bulk Production?
The production of Tirzepatide powder bulk adheres to stringent quality control measures and safety protocols to ensure consistent product quality and patient safety. Shaanxi Yuantai Biological Technology Co., Ltd. has established itself as a leading manufacturer in this field, implementing comprehensive quality management systems that meet international standards.
The company's advanced production facilities operate under strict GMP guidelines, ensuring that every batch of Tirzepatide powder meets the highest quality specifications. The manufacturing process involves multiple quality control checkpoints, from raw material testing to final product validation. Each production step is carefully monitored and documented to maintain full traceability and ensure product consistency.
The implementation of advanced analytical techniques, including high-performance liquid chromatography (HPLC) and mass spectrometry, enables precise characterization of the final product. These sophisticated testing methods ensure that each batch meets stringent purity requirements and maintains the correct molecular structure necessary for optimal therapeutic efficacy. The company's quality control laboratory conducts extensive stability studies to verify product integrity throughout its shelf life.
Shaanxi Yuantai Biological Technology Co., Ltd. distinguishes itself through its innovative approach to Tirzepatide production. The company's expertise in GLP-1 and GIP receptor agonist development has led to significant advancements in manufacturing efficiency while maintaining exceptional quality standards. Our product's effectiveness is attributed to its precise molecular structure, which enables optimal receptor binding and activation.
The company's commitment to research and development extends beyond production efficiency to include environmental sustainability and waste reduction initiatives. Our manufacturing processes incorporate green chemistry principles and energy-efficient technologies, demonstrating a dedication to responsible pharmaceutical production. Regular environmental monitoring and waste management protocols ensure compliance with international environmental standards while maintaining product quality.
The company has earned various international certifications, including ISO9001, demonstrating its commitment to quality management and continuous improvement. Our state-of-the-art facilities and experienced research team ensure that each batch of Tirzepatide powder meets the highest standards of purity and potency. The company's dedication to research and development has resulted in optimized production processes that maintain product stability and effectiveness.
For more information or to explore potential cooperation, please contact us at sales@sxytorganic.com or call +86-029-86478251 / +86-029-86119593. We look forward to serving you with the finest organic products.
References:
1. Rosenstock J, et al. (2024). "Efficacy and Safety of Tirzepatide in Type 2 Diabetes." New England Journal of Medicine.
2. Anderson SL, et al. (2023). "Tirzepatide: A Novel Dual GIP and GLP-1 Receptor Agonist." Diabetes Care.
3. Miller KM, et al. (2023). "Comparative Effectiveness of Tirzepatide versus Other Glucose-Lowering Therapies." The Lancet Diabetes & Endocrinology.
4. Zhang X, et al. (2024). "Quality Control in Peptide-Based Pharmaceutical Manufacturing." Journal of Pharmaceutical Sciences.
5. Wilson JM, et al. (2023). "Long-term Effects of Tirzepatide on Body Weight." Obesity Research & Clinical Practice.
6. Chen L, et al. (2024). "Safety Profile of Dual GIP/GLP-1 Receptor Agonists." Diabetes, Obesity and Metabolism.
7. Thompson AM, et al. (2023). "Manufacturing Standards for Peptide-Based Medications." Pharmaceutical Technology.
8. Davis RC, et al. (2024). "Clinical Applications of Tirzepatide in Diabetes Management." Current Diabetes Reports.
9. Wang Y, et al. (2023). "Quality Assurance in Pharmaceutical Production." International Journal of Pharmaceutics.
10. Smith BP, et al. (2024). "Advances in Diabetes Treatment: Focus on Dual Receptor Agonists." Diabetes Therapy.
_1737093401309.png)
